This treatment can protect vulnerable people from COVID. But many don’t know about it
Leanne Cook was glum but unsurprised when the tests confirmed what she and her doctors had expected: Even after three shots of a vaccine, she had no antibodies to protect her against COVID-19.
Her immune system had been hampered by the drugs she takes for her condition, a rare disease affecting her kidneys. As other vaccinated people began to let down their guard last year, Cook continued to minimize trips outside her home in Mission Viejo.
Then Cook heard about something that could plug those missing antibodies into her system — a preventive pair of injections called Evusheld. But health officials cautioned that there was only so much to go around.
Cook said that one medical provider told her, “‘We didn’t get any of this,’” she recalled. “And I’m like, ‘No, no, you guys got doses — I can see it on this website.’ ”
Cook ultimately secured the treatment in January, which finally gave her some antibodies to combat COVID-19 — and enough peace of mind to chance her first trip to a hair salon since the pandemic began. But it took networking, internet savvy and a costly consultation that landed her with an unexpected bill.
“I felt I was at their mercy to get a dose,” Cook said.
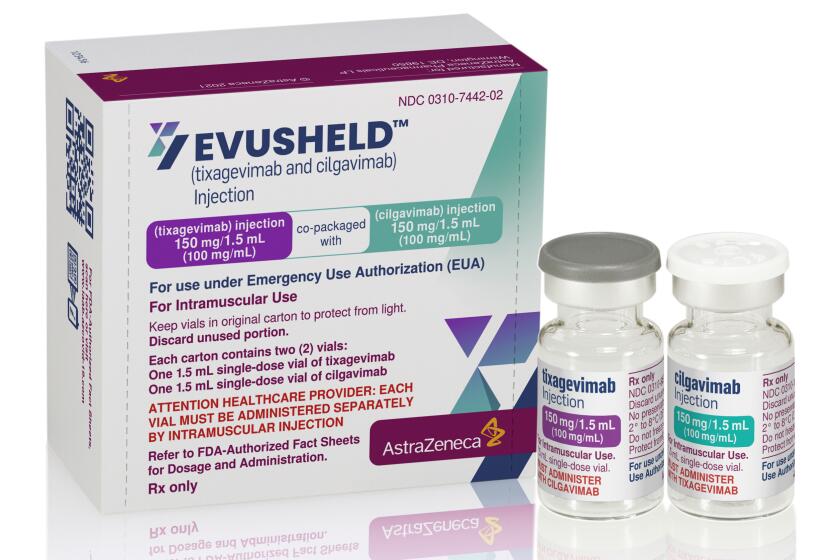
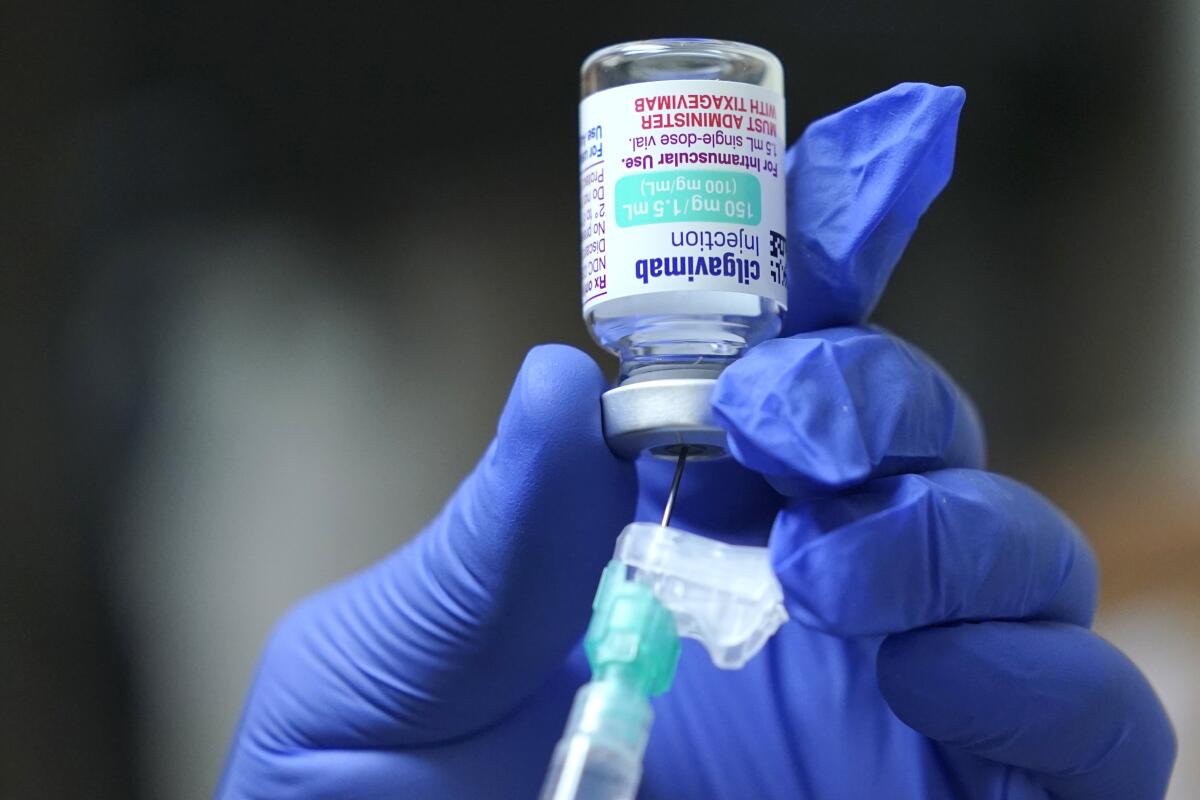
U.S. officials have authorized a COVID-19 antibody drug for people with serious health problems or allergies who can’t get adequate protection from vaccination.
Evusheld has been heralded as a way to armor people who remain highly vulnerable to COVID-19 even after vaccination. As government officials loosen masking requirements that have helped shield the immunocompromised, the preventive treatment has gained even more urgency for people who do not generate enough antibodies to gain protection from the COVID-19 vaccines.
The new treatment can “give them the antibodies that they essentially need in order to avoid getting admitted to the hospital” for COVID-19, said Dr. Krist Azizian, chief pharmacy officer for Keck Medicine of USC. “This could truly be lifesaving.”
But immunocompromised people and their advocates complain that scant awareness and a complicated process for allocating the limited supply have hampered the rollout of Evusheld, which is far from a household name even among the immunocompromised.
Many people who need it “don’t even know that it exists,” said Janet Handal, president and cofounder of the Transplant Recipients and Immunocompromised Patient Advocacy Group.
Because the antibody treatment has been sent to a limited number of medical providers, patients in the group have closely monitored a federal database that shows where Evusheld has gone. In Pasadena, Karol Franks said she spotted doses that had been sent to nearby hospitals and reached out to doctors to ask about getting it for her 36-year-old daughter.
“I said, ‘Hey, I see there’s Evusheld.’ And they’re like, ‘What’s Evusheld?’” Franks recalled.
One physician told her the treatment was still not available. Another said that her daughter — a kidney transplant patient who had no antibodies despite being vaccinated and boosted — was nowhere near the front of the line at his hospital. Franks persisted and her daughter Jenna eventually got the antibody shots elsewhere.
“If you’re a patient, you really need to stay on top of things,” Jenna Franks said. “You can’t just wait for the doctor to tell you what to do.”
So far, the federal government has agreed to buy 1.7 million courses of the treatment from AstraZeneca. As of late February, it had delivered nearly 600,000 doses for free to states and territories, according to the U.S. Department of Health and Human Services. Patient advocates have raised alarms about that number, pointing out that at least 7 million adults across the United States are estimated to be immunocompromised.
“It’s insane that there’s such a small amount for so many people,” said Michele Nadeem-Baker, who has chronic lymphocytic leukemia and is an advocate for people with blood cancers. She said she had become discouraged after discovering how few doses had been sent to the Boston institute where she is a patient.
“If you’re going to drop the mask mandate, please have enough Evusheld to go around for all the immunocompromised,” Nadeem-Baker said. If not, “I will continue to be locked up and missing life.”
Those worries mounted late in February, when the FDA started recommending twice as high a dosage to combat new sub-variants — a change that sent patients like Cook in search of another round of the shots. Physicians were already expecting to give eligible patients a second round of the treatment after six months.
Health and Human Services said it was working with health departments to “optimize their inventory” but added that, so far, states and territories had reported that only 20% of the allocated doses were being used.
In Los Angeles, several medical centers said that despite the limited supply, they had enough Evusheld to meet the demand they had seen so far, even with patients being referred from other providers.
“We really thought that the demand would be higher than it’s been,” said Dr. Caroline Goldzweig, chief medical officer for Cedars-Sinai Medical Network. More eligible patients are probably out there, “but doctors don’t necessarily know, ‘How do I look at my practice and find all the patients who are eligible for Evusheld?’ ”
Goldzweig said that, at first, Cedars-Sinai was limiting Evusheld to a group of especially vulnerable patients but soon made it available it to anyone who met federal criteria.
Heart transplant recipient Tania Daniels, who is a Cedars-Sinai patient, said that when she first began asking about the new treatment, “there was very little communication about Evusheld and its availability.”
Daniels said she was eager to get the shots after learning, through a study, that she had no antibodies or T-cells. In January, Daniels said, a nurse she knew told her that “it did not look good at that time.”
Then in February, she phoned to change an appointment, asked again about Evusheld, and finally heard that she could receive the shots, she said. Daniels, who retired from a corporate job to become a patient advocate, said that getting the antibodies had given her “tremendous relief.”
But “each state handles it differently. Each county handles it differently. Each institution handles it differently. And then you add on the fact that there’s not enough supply?” Daniels said. “It’s a mess.”
Dr. Brian Koffman, a retired family physician and cofounder of the CLL Society, which serves patients with chronic lymphocytic leukemia, said there should have been broad and proactive efforts to inform people that “this is how you get it. This is what to expect,” similar to the public education around COVID vaccines. Instead, “it was an afterthought.”
That lack of information has been compounded, he said, by a “haphazard” system in which different institutions have set different criteria for who is first in line for the treatment.
“You shouldn’t have to have a doctoral degree to get this stuff,” Koffman said.
Handal said some members of her group had scouted out centers where the treatment is more readily available and driven as many as 10 hours to get it for themselves or their children. Travel can be risky for immunocompromised people, she said, but “this is, for people, a life-or-death decision.”
Federal officials granted emergency use authorization for the drug in December, after a clinical trial found that Evusheld recipients had a 77% reduced risk of getting COVID-19. Under the federal approval, it can be given to people whose immune systems are compromised or who are medically unable to get the COVID vaccine — and who are not infected with or recently exposed to the virus.
Among the immunocompromised are people who have gotten organ transplants, who take medicine to suppress their immune systems. Last year, researchers found that transplant patients who were vaccinated were 485 times more likely than other vaccinated people to be hospitalized or die from breakthrough infections.
Remember all those problems we had with vaccine equity? Now they’re cropping up with new COVID-19 medications that are in short supply.
“We’ve got great vaccines,” said Dr. Dorry Segev, a transplant surgeon at NYU Langone Health who led that study. “But they’re not great for some people.”
Many patient advocates worry that the obstacles to getting Evusheld could disadvantage patients who do not use the internet or have little time to phone physicians or to bird-dog a government database.
Researchers have found that other forms of COVID monoclonal antibody treatments — those for people already infected or exposed — have been given less often to Black, Asian and Hispanic patients than white and non-Hispanic ones, according to a report recently released by the Centers for Disease Control and Prevention.
“I had to figure out everything on my own,” said Michael Stubbs, a retiree in Santa Barbara who has rheumatoid arthritis and other immune conditions.
“I’m white. I’m educated. I’m affluent. I have connections. My doctor has connections. ... That’s a privileged position to be in,” said Stubbs, who was among the first patients in Santa Barbara County to get the preventive antibody treatment. “My God, what about the folks ... who don’t have the resources I do?”
There can also be costs: Federal officials say the drug is free to eligible patients, but “there may be an associated administration fee.” Some patients have encountered significant charges from medical providers for administering the injections or vetting recipients for eligibility. Cook, in Mission Viejo, said she ultimately paid more than $1,100, the bulk of it for a brief consultation with a UC Irvine oncologist.
The bills came as a surprise, but “luckily for me ... my family is in the position where we can pay,” Cook said.
A UCI Health spokesman said its patients had not been billed for Evusheld itself, but providers “are permitted to charge for the handling and clinical administration related to the treatment” and that patients who are referred by outside physicians are evaluated by a UCI Health specialist to ensure the therapy is appropriate for them.
The mobile medical provider Concierge MD, which is based in Los Angeles, has been advertising Evusheld for $999. The cost includes screening by a medical provider, giving the patient the injections in their home, and monitoring them afterward for any allergic reactions, said Dr. Abe Malkin, owner of the company.
Malkin was unsure how much of that cost might ultimately be covered by health insurers. So far, “it’s not super popular,” Malkin said. “I just don’t think people know about it.”
In California, health officials aimed to address possible disparities through the allocation process: The antibody treatment was initially divvied up by region, then by county, with amounts tied to how much of the population was considered to be disadvantaged under a state metric, according to its public health department.
Los Angeles County, in turn, surveyed acute care hospitals in November to find out how many eligible patients they served, said Dr. Seira Kurian, who heads the COVID therapeutics effort at the county Department of Public Health. Kurian said that to decide how much goes to a hospital, the county also gauges inventory at each facility, checks in on its latest needs, and ensures that access points are spread across the county.
And at Cedars-Sinai, Goldzweig said an algorithm had been designed to prioritize patients from disadvantaged areas among those who were referred. But the demand has been so limited, so far, that the hospital system hasn’t used it.
HHS said it was “committed to doing everything we can to protect the health and wellbeing of all Americans, including immunocompromised people, which is why we’ve purchased as much product as the manufacturer can supply.”
An AstraZeneca spokesperson said, however, that the company still had additional doses available for purchase this year and was “committed to providing supply to all countries as quickly as possible where we have firm agreements.”
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.