Britain says study supports delaying second dose of AstraZeneca COVID-19 vaccine
- Share via
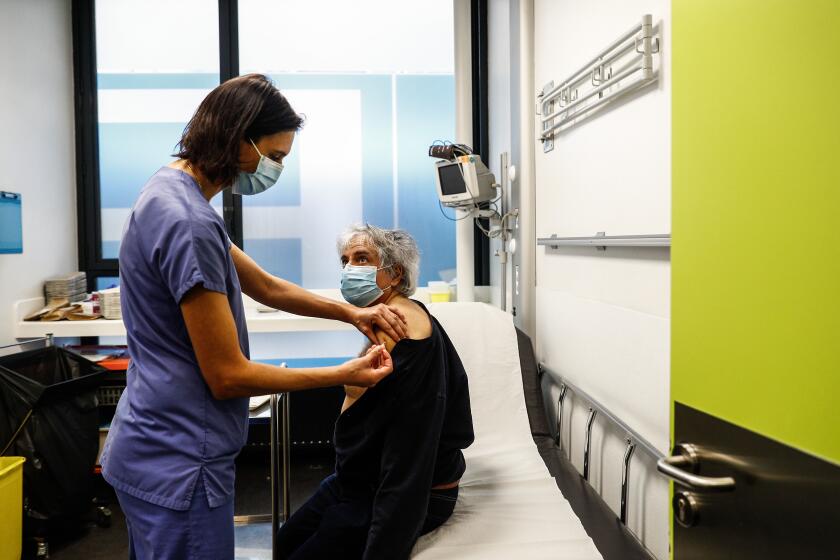
LONDON — Britain’s health chief has hailed a new study suggesting that a single dose of the Oxford-AstraZeneca COVID-19 vaccine provides a high level of protection for 12 weeks, saying it supports the government’s contentious strategy of delaying the second shot so it can protect more people quickly with a first dose.
Britain’s decision has been criticized as risky by other European countries, but Health Secretary Matt Hancock said Wednesday that the study “backs the strategy that we’ve taken, and it shows the world that the Oxford vaccine works effectively.”
Hancock’s comments came after Oxford released a study showing that the vaccine cut transmission of the coronavirus by two-thirds and prevented severe disease.
Mene Pangalos, executive vice president of biopharmaceuticals research and development at AstraZeneca, said no patients experienced severe COVID-19 or required hospitalization three weeks after receiving a first dose, and that efficacy appeared to increase up to 12 weeks after the initial shot.
“Our data suggest you want to be as close to the 12 weeks as you can” for the second dose, Pangalos said during a news conference.
The study has not been peer-reviewed yet, and it did not address dosing of the Pfizer-BioNTech vaccine, the other one in use in the U.K. Pfizer recommends that its shots be given 21 days apart and has not endorsed the British government’s decision to lengthen the time between doses.
San Diego County has California’s largest cluster of known cases of B.1.1.7.
But the Oxford research was greeted with excitement by British officials under pressure to justify their decision to delay the second dose.
“That reduction in transmission, as well as the fact there is no hospitalizations, the combination of that is very good news. And it categorically supports the strategy we’ve been taking on having a 12-week gap between the doses,” Hancock told Sky News.
Some countries, including France, have authorized the AstraZeneca vaccine only for use in people under 65, saying there is not enough evidence to say whether it works in older adults. Belgium has authorized it only for people 55 and under.
Yet one of the lead researchers on the Oxford vaccine project, Dr. Andrew Pollard, said “we expect it to be highly effective in older adults” and that more data should be available in the next few weeks.
The French are among the most reluctant people in the world to get a COVID-19 shot because of distrust of the government and past health scandals.
Pangalos noted that the European Medicines Agency had authorized the vaccine for use in all people over age 18.
“How individual countries decide to implement vaccines is ultimately up to them based on the vaccine supplies that they have,” he said.
Vaccine supply is a sensitive issue in the European Union, which is unhappy that AstraZeneca cut back on the number of doses that it plans to supply to the EU in the near term. The company said last month that it planned to cut initial deliveries within the EU from 80 million doses to 31 million doses because of reduced yields from its manufacturing plants in Europe.
It has since agreed to supply 9 million additional doses to the 27-nation bloc, whose leaders are facing criticism over what is perceived as slow progress in inoculating the population.
The 27-nation EU is coming under fire by residents for the slow rollout of the bloc’s coordinated COVID-19 vaccination campaign.
Britain has Europe’s deadliest coronavirus outbreak, with more than 108,000 deaths, and is in its third national lockdown as authorities try to contain a new, more transmissible virus variant first identified in southeast England.
Other variants are also a concern. Public health officials in England are going door to door, trying to test all adults in eight targeted communities in an attempt to stop a new strain first identified in South Africa from spreading further.
So far, 105 cases of the variant have been identified in Britain, 11 of them in people with no links to overseas travel. Scientists say there is no evidence the South African strain is more serious than the original coronavirus but it may be more contagious. There are also concerns that current vaccines may be less effective against that variant because it contains a mutation of the virus’ characteristic spike protein that existing vaccines target.
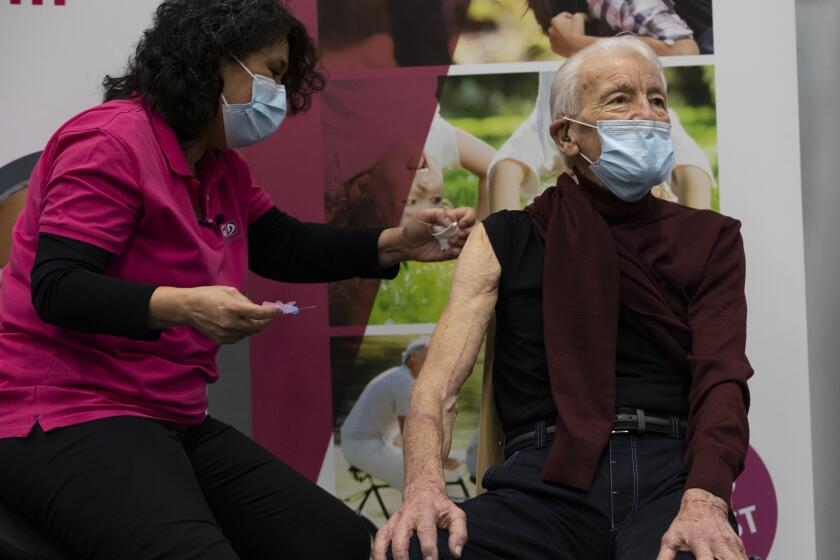
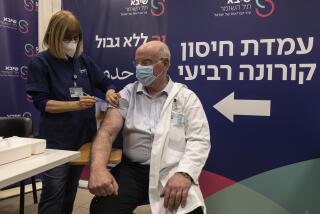
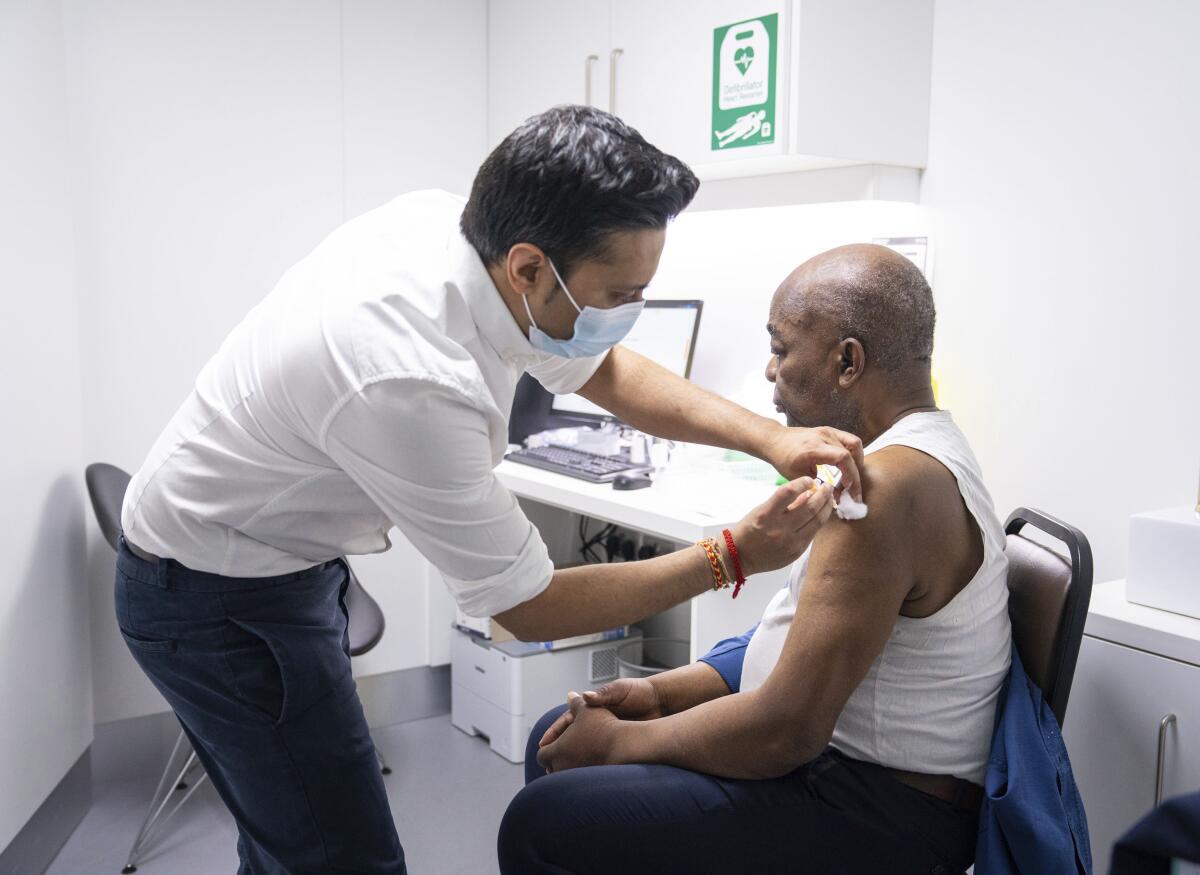
That is a worry as Britain races to vaccinate its own population against the virus. Almost 10 million people in the U.K. have received the first of their two shots, including the bulk of people over 80 and those in nursing homes.
Pollard said Oxford scientists believe the AstraZeneca vaccine will continue to offer protection against new variants of the coronavirus, although they are still waiting for data on that.
Even if the virus adapts, “that doesn’t mean that we won’t still have protection against severe disease,” Pollard said.
“If we do need to update the vaccines, then it is actually a relatively straightforward process. It only takes a matter of months, rather than the huge efforts that everyone went through last year to get the very large-scale trials run,” he told the BBC.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.