The Silicone Controversy : Health: Activists take offensive against breast implants, alleging health risks; many doctors, manufacturers say they’re alarmists.
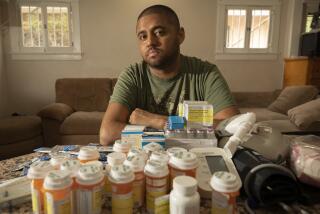
For years, Marie Walsh, a Laguna Hills mother of eight, watched her health deteriorate. She was plagued with fatigue, abdominal pains, irregular menstrual periods and night sweats.
Then one morning in February of 1984, she awoke and found the front of her nightgown damp with a bloody gel. A surgeon found that an implant in her left breast had ruptured, spilling silicone gel over the breast cavity. He cleaned out as much of the gel as he could and put in a new implant.
But Walsh’s problems worsened.
She needed multiple surgeries to replace later implants that hardened uncomfortably and to remove silicone-filled tumors . She starting developing a variety of debilitating diseases: rheumatoid arthritis that made it painful for her to walk; scleroderma that tightened her skin and made her hollow-eyed, and chronic lymph, kidney and liver problems.
“I’m disintegrating,” she said. “It’s just a question of time (as to) what is going to go next.”
Walsh and her doctor are convinced that silicone gel from the implants has migrated through her body and caused chaos in her immune system.
Earlier this year, Walsh won an $80,000 settlement against the manufacturer of the ruptured breast implant and that publicity has spun her into the center of an emerging nationwide network of women who allege that implants pose health hazards that are being kept a secret from consumers.
She and other activists are crashing seminars on breast augmentation, courting newspaper and television reporters and filing lawsuits to draw attention to their plight.
Their fears are considered irrational by manufacturers of breast implants and most plastic surgeons, who contend that the devices’ benefits--primarily that of boosting a woman’s self-esteem--outweigh the risks. They say there is no scientific evidence to prove any link between breast implants and cancer or diseases that attack the immune system.
Dr. Garry Brody, a clinical professor of plastic surgery at the University of Southern California and chairman of the society’s plastic surgery device council, complained that the women in the Command Trust Network are discouraging women who would benefit from breast implants by “spreading panic.”
Brody said a USC study is following more than 3,000 women in Los Angeles County who have had silicone breast implants and discovered so far that they have no higher incidence of cancer than the general public.
But breast implants are becoming increasingly controversial.
Safety concerns have prompted the U.S. Food and Drug Administration and its Canadian counterpart to call for further investigations and to collect test data from manufacturers.
Critics of breast implants have in their corner a Ralph Nader-founded advocate organization, Public Citizen Health Research Group, which has demanded that implants be taken off the market and has sued the FDA for access to manufacturers’ records.
The stakes are high. An estimated 2 million U.S. women have had breast implants, which are being performed at a rate of about 130,000 a year. About 85% of breast implants are cosmetic. The rest are performed to reconstruct breasts after cancer surgery.
Said Dr. William Shaw, chief of the division of plastic surgery at UCLA Medical Center: “It could be a bigger problem waiting to be confirmed or a smaller problem with a great deal of hysteria.”
At the center of the controversy are devices that look like sacks made of a rubbery silicone skin and filled with silicone gel.
They were designed by two plastic surgeons from Houston looking for an alternative to plastic sponge implants, used since the 1930s, which were unacceptable because they made the breast very hard and frequently caused allergic reactions.
Doctors also were injecting silicone liquid directly into women’s breasts, in some cases causing severe health problems, but the FDA stopped that practice in the early 1960s.
The first silicone-gel devices were implanted in 1963, and they have become especially popular in recent years as fashion and films have stressed the larger-breasted look. Advertisements in women’s magazines and newspapers have enticed women with the purported ease of the procedure, which costs $2,000 to $5,000.
Silicone gel is used in the implant to give a natural feeling to the breast; the sack is designed to prevent the gel from getting into body tissue.
However, it quickly became obvious that the device had problems. Patients complained about excessive scar tissue building up around the implant, making the breast unnaturally hard and causing pain. This condition occurs in 30% to 50% of breast-implant patients, many of whom have repeated surgeries to try to correct it.
Also, it is generally conceded that implants can hamper efforts to detect breast cancer through mammography.
Of far greater dispute is whether silicone gel, some of which leaks out of all implants, can damage a woman’s health. While the medical community once considered silicone “inert” within the body, more recent research indicates that that may not be the case.
There is additional concern about a type of silicone implant surrounded by a covering of polyurethane foam that is intended to reduce the scarring.
The foam degrades in the body and some studies have shown that under certain laboratory conditions, the disintegrating foam emits diamino toluene diamene, a known carcinogen in animals.
The FDA is beginning to look closer at these issues and demand more information from manufacturers.
The agency in 1988 determined that breast implants pose “a potential unreasonable risk of injury,” but it is still drafting the testing requirements that manufacturers will have to meet. For now, implants can be sold without passing any safety tests specified by the FDA.
The FDA acknowledges that even if preliminary tests show breast implants to be safe, it may be 10 or 15 years before there is enough data to know for certain if implants increase the risk of cancer or immune-system diseases.
Despite her lingering illnesses, Marie Walsh still carries implants in each of her breasts.
“Every doctor has told me it won’t make a difference” in her health at this point whether they are removed or not, she said. But recently, she decided to take them out after consulting with a new physician.
“Her problem is induced by the silicone, and if she takes it out, there is at least a 50% chance that she will improve,” said Dr. Gharoon Panahi, a Westminster rheumatologist.
“I’d give anything to have my life back,” she said. “I would rather be flat-chested and have my health and vitality.”
Walsh, who has been forced by ill health to quit a career in securities sales and go on permanent state disability, is devoting much of her energy to organizing a support group in Orange County for women with similar problems.
She is assisted by Marsha Chambers, a Tustin resident who got in touch with Walsh after reading a newspaper story about her legal settlement. Chambers suffers from hepatitus and other ailments she blames on the breast implants that she had removed in June.
Together the women placed a tiny ad in a local Tustin newspaper that attracted 40 more women who now meet regularly to discuss what to do about their health and how to warn others who may be thinking about enlarging their breasts.
They have scoured medical libraries for information about the hazards of breast implants and they show up at public seminars on breast augmentation to distribute flyers.
Their group, HAD (after “Human Adjuvant Disease”--the medical term for the mosaic of symptoms they share), is linked with a nationwide activist organization called Command Trust Network.
Command Trust Network was founded last year by Kathleen Anneken of Covington, Ky., and Sybil Goldrich of Beverly Hills, both of whom had a common history of breast-implant problems and a desire to reach out to others.
“We decided an organization had to be established to provide the kind of information not routinely given in physicians’ offices but information that we believed was essential for a woman to make a completely informed decision about what she was putting in her body,” Goldrich said.
She complains that so far, Maryland is the only state in the country that has enacted legislation requiring physicians to inform women of the risks of breast implants through a standardized brochure.
Joseph Arcarese, director of the FDA’s office of training and assistance for medical devices, said two years ago an FDA advisory panel held hearings and determined that “patients are poorly informed.” In response to the panel’s request, he said the FDA has created committees representing consumers, physicians and the medical industry to write informational pamphlets that could be given to women considering breast enlargement or reconstruction after mastectomy.
But as yet, he said, the members of the committees, which include representatives of Command Trust Network, have failed to reach a consensus on what should be said in the pamphlets, which are in their fifth draft.
In addition, a growing number of women with breast-implant problems are suing the manufacturers. A small number of trial attorneys are taking on the cases, while Public Citizen is acting as an informational clearinghouse for plaintiffs.
Public Citizen estimates that there are at least 200 product-liability lawsuits pending against silicone breast-implant manufacturers.
Denise Dunleavy, a Manhattan lawyer, said, “I have eight cases and I could probably take hundreds more if I wanted to. There are so many women out there looking for attorneys that it is frightening.”
In 1984, a federal jury ordered Dow Corning Corp. of Midland, Mich., to pay $1.5 million in punitive damages and $211,000 in actual damages to Maria Stern, a Nevada woman whose breast implants made by that firm leaked silicone into her body after she had a mastectomy. Before the case was heard on appeal, Stern and Dow Corning reached an undisclosed settlement.
Dan Bolton, a Redwood City attorney who worked on the Stern case, said he has since taken the cases of 10 to 15 other women with breast-implant problems. He said in five of those cases, the plaintiffs have received out-of-court payments to drop the litigation. So far, none of the cases have gone to trial.
Bolton said that routinely, as part of such settlement agreements, the manufacturers insist that the size of the settlement payment and the documents that the plaintiffs have gathered to build a case against the manufacturers be sealed from the public.
Said Bolton: “The purpose of that is to make it very difficult and financially burdensome for any other plaintiff attorney to take on a similar case (because it) precludes plaintiff attorneys from sharing information.”
Advocates of breast implants contend that litigation is triggered by greedy lawyers and a small group of hysterical women who are ill and looking for someone to blame.
The American Society of Plastic and Reconstructive Surgeons recently reported the results of a survey of 592 women with breast implants showing that 92.5% were satisfied, while 82% said without a doubt that they would choose to have the surgery again.
Also, while a study by Dow Corning showed that silicone produces cancerous growths in rats, Dow Corning and the FDA agree that this is not the kind of cancer to which humans would be susceptible.
Robert Rylee, vice president of Dow Corning Corp., a major manufacturer of silicone gel-filled implants, said: “There is too much emotionalism that gets into this thing. What we really need is more understanding and true science. I think the medical community and manufacturers are doing everything they can to identify what can be done to increase our scientific knowledge.”
Rylee said that Dow Corning is trying to develop a national epidemiology study to determine if there is any link between silicone breast implants and immune-system disorders. A major difficulty, he said, is that the diseases in question--lupus, scleroderma and rheumatoid arthritis--have always been most prevalent in women between 20 and 55, the same age group most likely to get breast implants.
“None of our scientific consultants or internal scientists or physicians have yet been able to show there is a causal relationship,” he said.
The most controversial implants are those covered with polyurethane foam that are marketed by Surgitek, a Paso Robles subsidiary of Bristol-Myers Squibb Co., the New York-based pharmaceutical and consumer-products giant.
Garry L. Carter, vice president-general manager of Surgitek’s plastic surgery division, said the polyurethane-coated implant “is probably the fastest-growing product in the industry,” currently representing about 20% of all implant sales in the United States.
However, Dr. Richard Caleel, president of the American Academy of Cosmetic Surgery, said: “There are a lot of cosmetic surgeons who do not use polyurethane-covered implants because they are not sure about what happens to the polyurethane.”
A study by Chris Batish, a biomaterials scientist and professor at the University of Florida, showed that the kind of polyurethane used by Surgitek is not favored for medical applications because of its tendency to disintegrate in the body. Once that happens, Batish said in a recent interview, “no one is sure where it goes.”
In addition, Batish said he found that in the laboratory, he could break the foam into chemical components that included the animal carcinogen TDA.
Surgitek counters that the laboratory conditions used by Batish were much harsher than the environment inside the body. The company says studies that more closely approximate human biology show that the foam degrades very slowly and passes through the body without causing harm.
Surgitek also says its research shows that the amount of TDA produced when the foam disintegrates is medically insignificant.
The Canadian government has recently completed its own laboratory test of the foam and found no release of TDA, said Irwin Hinberg, acting chief of the research and standards division of Canada’s Health Protection Branch.
However, while neither the Canadian government nor the FDA believe that there is evidence to outlaw polyurethane-coated implants, both are advocating more studies.
“Everybody is extrapolating (from laboratory tests). Nobody has tested what happens in the body,” Hinberg said, adding that studies are under way to measure chemicals being produced in women who have had implants.
But a few doctors and scientists believe there is already more than enough information about the dangers of breast implants to raise a red flag.
Dr. Douglas R. Shanklin, a pathologist at the University of Tennessee, calls the silicone implants “an immunological and biological disaster waiting to happen.” He said that some women with implants “go 5, 10, 15 years with no problem and then all hell breaks loose.”
Shanklin, who said he has seen silicone in the tissue taken from the thyroid glands and spleens of women who have had breast implants, said: “I think they should be taken off the market pending resolution of these problems.”
He added that the various immune-system diseases that women are reporting can be serious. “These are fatal diseases in time,” he said. “Lupus destroys the kidneys and scleroderma can effect the heart and lungs.”
Michael Rensch, an assistant professor in the department of engineering mechanics at the University of Nebraska, said that implants are covered with a “highly elastic silicon rubber that can quite easily tear.” He said he has “examined over 500 deflated breast implants” and has been retained by plaintiff attorneys in 50 to 60 cases to render opinions regarding manufacturing and design defects.
“From all the pain and suffering I have seen, I wouldn’t recommend anyone to use these devices from anyone or any company,” Rensch said.
UCLA’s Shaw said that in the last year, he has seen a dozen women with breast implants who also have arthritis and various other immune diseases.
“Right now, it is difficult to tell who has severe symptoms from the implants and who would have had them anyway. The causes of the diseases are a mystery,” he said. Nonetheless, “you have to admit it looks awfully suspicious. . . . This is a subject I think should be looked at. We shouldn’t brush these people off.”
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.