Column: When should you get excited about a coronavirus vaccine? Not yet
Somewhere there are eight individuals who can take pride in having contributed to a nearly 1,000-point leap in the Dow Jones industrial average on Monday, as well as a nearly $5-billion one-day leap in the value of an obscure money-losing biotech company called Moderna Inc.
Those eight are the subjects whose early results in a preliminary clinical trial of a COVID-19 vaccine were reported by Moderna on Monday morning. The main goal of the trial was to determine if the vaccine made the subjects seriously ill. For these eight, it didn’t; that’s good news. It also produced useful antibodies in those subjects.
But full results for the other 37 subjects in the Phase 1 trial haven’t been reported. Nor have Moderna’s results been subjected to peer review.
The totality of the science tells us that this is the right antigen and we should be protective.
— Dr. Tal Zaks, Moderna Inc.
Still, the scant data reported by Moderna were enough for investors’ hearts to leap up, as the poet Wordsworth recalled reacting upon beholding a rainbow in the sky. Moderna shares closed Monday at $80 in Nasdaq trading, a gain of $13.31, or nearly 20%.
One hates to be the bearer of bad news, which in this case means placing Moderna’s announcement in context, but the truth is that Moderna hasn’t announced a vaccine and the path to developing one remains long and tortuous.
Whether Moderna’s early trial will result in a vaccine, or when, remains highly uncertain; most drugs that deliver promising Phase 1 clinical trial results end up failing in the final analysis. There’s no reason to expect that this one will necessarily buck the odds. Moderna’s vaccine is one of many being tested.
The U.S. is suffering shutdown fatigue because the COVID-19 war has stagnated.
Evidence that the trial vaccine produced antibodies in humans that protect against the coronavirus in the lab is encouraging. But it doesn’t yet warrant terming the vaccine a success. Much could go wrong as the drug undergoes broader testing and its actual protective effect is measured. Then there’s the challenge of manufacturing any vaccine that has been approved and distributing it broadly enough to secure widespread immunity.
What would be grounds for genuine enthusiasm? Certainly trial results showing that a vaccine actually blocked infection by the coronavirus in humans without causing side effects that could sicken patients or even make them more susceptible to infection — outcomes that have happened with vaccines against other diseases.
But that would require large-scale randomized, blind trials — those in which neither the patients nor the researchers know who received the test vaccine and who a placebo. Such trials will be difficult to conduct and not likely to start until the second half of this year at the earliest.
The market’s reaction to Moderna’s news says less about the scientific significance of the announcement than about how much the world, investors or not, is grasping at, or groping for, the barest smidgen of good news in the fight against COVID-19. In scientific terms, of course, the issue is whether this smidgen of good news has legs.
For Moderna, the reaction was spectacular. The Cambridge, Mass., firm has never recorded any revenue from the sale of drugs, only from research grants and the licensing of its early-stage technology to other companies. It has lost more than $1.2 million over the last three years. So a lead role in the quest for a COVID-19 vaccine would boost its fortunes.
Even if Moderna did manage to perfect a vaccine, it’s not entirely clear how much it might profit from the discovery. If a vaccine does appear, the drug industry will be under tremendous pressure to limit its profits in order to ensure nearly universal access to the product.
Clinics with unproven stem cell treatments are already targeting COVID-19 fears.
But the company is dependent on a steady stream of positive news to maintain its stock price, which had nearly quadrupled this year even before Monday’s announcement. Any inkling of a pessimistic result would take all that froth out of the share price.
Moderna’s announcement, it’s proper to observe, came only days after former board member Moncef Slaoui was appointed to lead the White House’s Operation Warp Speed vaccine development initiative. Slaoui resigned from the board to take the unpaid government position. He still holds an estimated $10 million in Moderna stock options, provoking government watchdog groups to warn that he could be embroiled in a conflict of interest as long as he holds the options.
Let’s put Monday’s data in context. Moderna’s announcement didn’t cover such an essential question as whether its vaccine candidate would actually protect humans against the coronavirus that causes COVID-19. It couldn’t, because that wasn’t an outcome the trial was designed to establish. A Phase 3 trial, which would involve many thousands of human subjects, won’t even be launched until July at the earliest, and only then if all intermediate tests go well.
Phase 1 drug trials typically involve a very small number of subjects and are aimed at determining the short-term safety of a drug. In this case, do the subjects tolerate the vaccine — does it make them sick or have troubling side effects — and does it produce antibodies in the subjects’ blood?
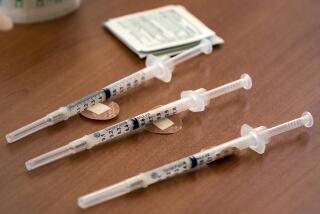
Moderna, working with the National Institutes of Health, recruited 45 healthy subjects ages 18 to 55 for the Phase 1 trials. They were divided into three groups receiving doses of 25, 100 and 250 micrograms in two shots 28 days apart.
Thus far, the available results on key antibody production cover only the first eight subjects. There’s no question that those results and others from the preliminary trial were encouraging, as far as that goes. Moderna says that only one of the 45 test subjects experienced redness around the injection site, and only three of the subjects given the highest dose experienced flu-like symptoms. But those disappeared after a day or so.
The finding causing the most excitement was that by 43 days after the first injection, the vaccine appeared to produce “neutralizing antibodies” that appeared to block the coronavirus in four subjects each receiving the lowest doses. Indeed, Moderna says the levels of those antibodies in the eight test subjects were higher than those found in COVID-19 patients who have recovered from the virus.
The America that condones mass murder of children is allowing the coronavirus to spread.
In the search for a COVID-19 vaccine, neutralizing antibodies are the ballgame. They’re the substances that would protect humans from infection. They’ve been shown to work against the novel coronavirus in mice.
In a conference call Monday morning with Wall Street investment analysts, Moderna executives struggled to hold back their optimism. “The totality of the science tells us that this is the right antigen and we should be protective,” Tal Zaks, Moderna’s chief medical officer, told the analysts.
Zaks said the results answered at least the question of whether a vaccine was even possible against the coronavirus. “These data today take off the table the risk of not being immunogenic or the risk of the antibody type being wrong,” he said. “No, it works, and you’ve seen a demonstration of neutralizing activity.”
But he also said that it may yet be difficult to demonstrate the vaccine’s safety and efficacy in a large randomized trial. In part, that’s because the most important trial, Phase 3, would have to be conducted in coronavirus hot spots where it would be easier to determine that those receiving the vaccine are immunized against the virus.
“If I vaccinate a whole bunch of people — it doesn’t matter how many — if there is no circulating virus in the places where I choose to vaccinate,” Zaks said, “it will be a long time before we know.”
Work on possible vaccines is proceeding worldwide at unprecedented speed. In the U.S., the effort is being overseen by the National Institutes of Health, which has created a system allowing research to proceed along several streamlined paths at once.
“A public-private biomedical research partnership of this scope and scale has never before come together in such a short time frame,” NIH Director Francis S. Collins observed in a statement published Monday. But he cautioned that some aspect of vaccine development can’t be short-circuited, noting that “the earliest possible distribution [is] predicted to be 12 to 18 months away.”
That’s the view of Anthony Fauci, the respected director of the National Institute of Allergy and Infectious Diseases, a unit of the NIH, who offered senators the same timeline in recent testimony.
In these unprecedented conditions, it’s understandable to seize on hope anywhere we find it. But it’s also wise to remember that lots of things can go unexpectedly awry, especially under unprecedented conditions.
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.