U.S. signs $1.95-billion deal with Pfizer for COVID-19 vaccine doses

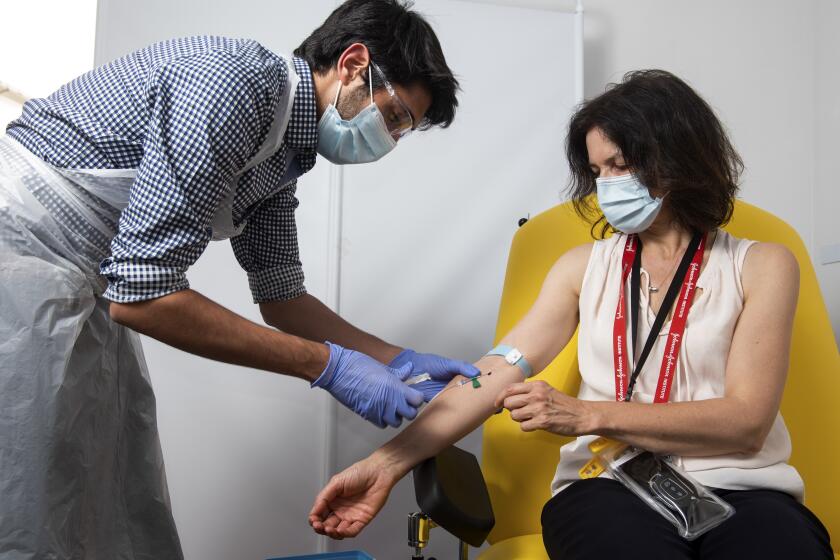
WASHINGTON — The U.S. has signed a $1.95-billion deal with Pfizer for delivery in December of the first 100 million doses of a COVID-19 vaccine the pharmaceutical giant is working to develop, Health and Human Services Secretary Alex Azar announced Wednesday.
The U.S. could buy an additional 500 million doses under the agreement, Azar said.
“Now those would, of course, have to be safe and effective” and be approved by the Food and Drug Administration, Azar said during an appearance on Fox News.
Pfizer and BioNTech announced separately that the agreement is with HHS and the Defense Department for a vaccine candidate the companies are developing jointly. It is the latest in a series of similar agreements with other vaccine companies.
The new deal is part of President Trump’s Operation Warp Speed vaccine program, under which multiple COVID-19 vaccine hopefuls are being developed simultaneously. The program aims to deliver 300 million doses of a safe and effective COVID-19 vaccine by January, according to HHS.
Under the initiative, the government will speed development and buy vaccines — before they are deemed safe and effective — so that the medication can be in hand and quickly distributed once the FDA approves or authorizes its emergency use.
Scientists say their experimental coronavirus vaccine has been shown in an early trial to prompt an immune response in hundreds of people.
Pfizer and BioNTech said the U.S. will pay $1.95 billion upon receipt of the first 100 million doses, following FDA authorization or approval. Americans will receive the vaccine for free, the companies said.
Azar said the contract with Pfizer and BioNTech brings to five the number of potential coronavirus vaccines that are under development. Nearly two dozen are in various stages of human testing around the world, with several entering final phases to prove if they are safe and effective.
Pfizer is finishing an earlier stage of testing to determine which of four possible candidates to try in a larger, final study.
As early as next week, a vaccine created by Moderna and the National Institutes of Health is set to begin final-stage testing in a group of 30,000 people. A number of large studies in the U.S. are planned to begin each month through the fall in hopes of eventually having several vaccines to use.
The funds will allow the company to conduct advanced human studies and establish manufacturing to deliver 100 million doses as soon as late 2020.
A few other potential vaccines in other countries have also begun smaller late-stage studies.
Trump said Tuesday at a briefing that “the vaccines are coming, and they’re coming a lot sooner than anyone thought possible, by years.”
But other countries are also scrambling to get their hands on a vaccine for COVID-19, which has killed more than 617,000 people, according to a tally kept by Johns Hopkins University.
Britain announced Monday that it had secured access to another 90 million experimental COVID-19 vaccines made by Pfizer and others, a move some campaigners warned could worsen a global scramble by rich countries to hoard the world’s limited supply of COVID-19 vaccines.
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.