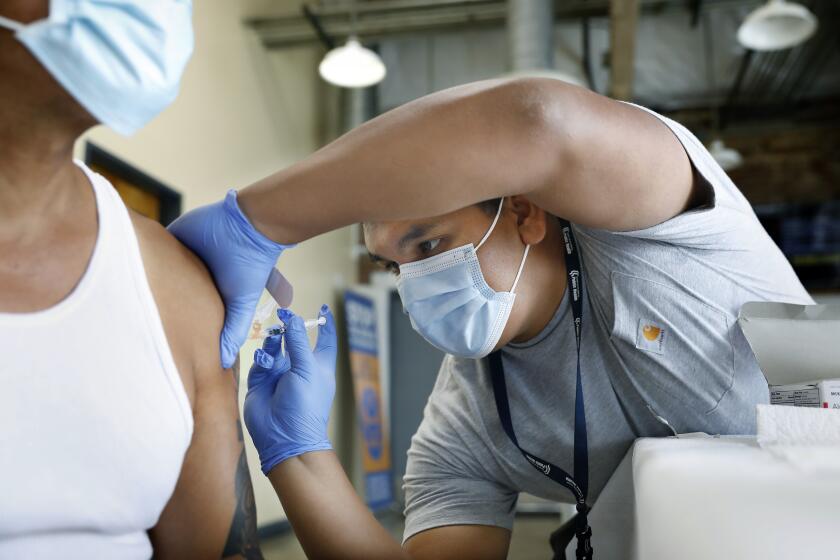
COVID vaccine should be updated to target XBB strain, FDA committee says
With an eye toward enhancing protection against the coronavirus, which is still evolving and circulating, federal health advisors said Thursday that the next round of COVID-19 vaccines should be updated to target one of the XBB strains currently dominating the viral landscape.
The unanimous recommendation from the Food and Drug Administration’s vaccine advisory committee follows that of agency staff, who in a memo acknowledged that while older vaccine formulas can still help stave off severe disease, “protection wanes with time and is reduced against subsequent waves of variant viruses.”
It also sets the stage for the next phase in the nation’s years-long inoculation campaign, an effort that’s been widely credited with helping end the pandemic emergency, even as public interest in additional shots continues to wane.
This would mark the second wide reformulation of COVID-19 vaccines, following the release of a “bivalent” shot last September. That version offered protection against both the then-dominant Omicron subvariants and the ancestral coronavirus strain, but the FDA panel recommended not including the latter this time.
The committee’s recommendations are not binding, though the FDA typically follows the advice. The Centers for Disease Control and Prevention also is expected to weigh in on the matter, with its Advisory Committee on Immunization Practices scheduled to discuss COVID-19 vaccines on June 23.
Eligibility and timing of any newer-recipe vaccines are yet to be officially determined. In a recorded audio statement, Dr. Peter Marks, the FDA’s vaccine chief, said the agency will “make a decision quickly” with the goal to have the updated vaccine available by September.
Coronavirus infections have been found in at least 32 animal species in 39 countries. Some can transmit the virus to humans.
Data suggest that using the latest dominant coronavirus strain that more closely matches current circulating Omicron sublineages “is warranted for the 2023-2024 vaccination campaign,” according to FDA staff.
“I think that we need a better vaccine. We should be updating it, and I think it’s pretty straightforward,” said Dr. Eric Rubin, a committee member and infectious disease expert at the Harvard T.H. Chan School of Public Health.
Some experts and officials have hypothesized that the cadence of COVID vaccinations will eventually mirror that of flu: one shot updated and administered annually. But it’s unclear whether that time has arrived.
Also unknown is whether COVID will — like flu — settle into a fairly predictable seasonable pattern that would make it easier to time vaccines for optimal protection. Although coronavirus activity has ramped up every fall and winter since 2020, there were also upticks during the spring or summer each year.
The appetite for another COVID shot also remains to be seen. Statewide, only 26.2% of eligible Californians have gotten a bivalent vaccine in the nine months since that formulation became available.
That apathy may now be more acute, with the emergency phase of the pandemic over and COVID-19 no longer at top of mind for many.
One in 10 people infected with the coronavirus during the Omicron era suffered from long COVID, indicating the syndrome remains a notable threat.
Virtually all versions of the coronavirus currently circulating in the U.S. are descendants of the original Omicron variant that walloped the nation in late 2021 and early 2022. But among those, the XBB family — which includes XBB.1.5 and XBB.1.16, unofficially dubbed “Kraken” and “Arcturus,” respectively — is dominant almost to the point of monopoly.
According to the most recent estimates from the CDC, XBB sublineages constitute nearly 99% of circulating coronavirus in the U.S., displacing the BA.5 subvariant that dominated the nation for much of last year.
Data are accumulating that “suggest that a vaccine composition that more closely matches circulating virus strains can significantly improve vaccine-induced immunogenicity and protection,” FDA staff said.
“It’s a great idea to base the vaccine on what’s circulating right now,” UC San Francisco infectious disease expert Dr. Peter Chin-Hong said in an interview. “And XBB is the main game in town. It’s been around for some time now. It probably will continue to be dominant.”
The end of the federal government’s health emergency has left COVID-conscious Americans feeling vulnerable. Here are some steps you can take to protect yourself.
Given patterns over the last year, the coronavirus has been evolving more incrementally, Chin-Hong said. So he said picking a vaccine formula based on a current dominant subvariant is likely to still be helpful heading into the fall and winter, when some officials and experts expect a degree of resurgence.
The updated vaccine, the FDA panel agreed, should be a “monovalent” shot focused on XBB.
Future iterations of the vaccines need not protect against the ancestral strain, scientists say. A World Health Organization technical advisory group said in May there is no longer a need for that since the original version of the coronavirus that emerged in late 2019 in Wuhan, China, is essentially extinct.
Committee members on Thursday appeared supportive of updating vaccines specifically to target XBB.1.5.
In the U.S., XBB.1.5 made up the majority of circulating virus in the early spring but now is estimated to constitute about 40% of specimens, CDC figures show. The upstart XBB.1.16 is gaining ground and is now estimated to make up 18% of cases nationwide.
In Los Angeles County, XBB.1.5 accounted for 66% of analyzed specimens as of early May.
Though this past winter saw nowhere near the same degree of devastation as the pandemic’s first two winters, the nation and California still saw an uptick in coronavirus-positive hospitalizations, as well as a considerable number of deaths. From October through early June, about 75,000 COVID-19 deaths have been reported nationally. That’s more than double the average annual number of flu deaths in the decade preceding the COVID-19 pandemic.
In order to move through a world where the coronavirus is endemic, we need a reliable way to assess our individual level of immunity. Here’s how we can.
There’s emerging consensus regarding the value of periodically updating COVID-19 vaccines.
“In order to improve protection, in particular against symptomatic disease, new formulations of COVID-19 vaccines should aim to induce antibody responses that neutralize XBB descendant lineages,” the WHO advisory group said.
And it’s possible the vaccines will need to be reformulated again, depending on how the coronavirus continues to evolve.
“This is not going to be the final formulation for this vaccine forever more,” Marks said during Thursday’s committee meeting. “It will probably require another update at some point.”
In terms of figuring out a near-term vaccine formulation, however, the WHO advisory group noted XBB.1.16 has very small genetic and antigenic differences from XBB.1.5. “The spike antigens of both of these lineages are genetically and antigenically very closely related, with only two amino acid differences between” them.
Updating the vaccination formula ahead of last autumn and winter season was helpful, officials said.
“Observational data indicated that [last year’s updated] COVID-19 vaccines provided improved protection from COVID-19 caused by sublineages of Omicron, including the BA.4/BA.5 sublineage, compared to the original vaccines,” FDA staff said in their statement.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.