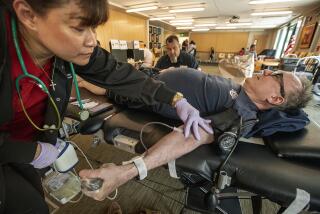
Opinion: Will new blood donation rules finally stop stigmatizing gay men?
As a sophomore in college in 2011, I was deferred from donating blood for being a gay man. I was confronting the homophobia built into the FDA’s blood donation ban for men who have sex with men.
After imposing that rule in 1985 and making a few minor revisions since, the FDA is at long last considering meaningful updates to consider donors based on their sexual behavior regardless of their orientation. The new regulations would allow gay and bisexual men to donate blood — if they are monogamous. The proposal would also for the first time defer anyone from donating if they have had new or multiple partners in the past three months and had anal sex during that time.
It’s progress that officials are revisiting policies that have enshrined stigma and prejudice. The changes would open the door to many gay and bisexual men who were previously — and wrongly — excluded, helping shore up the U.S.’s lagging blood supply.
Donating blood may be the best holiday gift this season, as the United States faces a shortage.
But we need further changes. The new policy’s narrow focus on anal sex and monogamy still fails to assess sexual risk for HIV accurately, and it continues to stigmatize people based on their sexual activity.
First, as the FDA’s proposal highlights, not all types of sex are equal when it comes to HIV risk. The highest such risk is from unprotected anal sex with an HIV-positive partner who has a detectable viral load. But risk differs significantly based on position — whether someone is the receptive or insertive partner. Unprotected receptive vaginal sex and unprotected insertive anal sex have a comparable risk of HIV transmission, meaning a policy focus only on the latter reflects some subjective judgment of sexual activity risks.
Monogamy is also not necessarily a reliable means of preventing sexually transmitted infections, given the reality of sexual activity outside of presumed monogamous relationships. Research shows that individuals in consensual nonmonogamous (e.g. open) relationships are actually more likely to get HIV and STI testing and use condoms than those who report monogamy. They may therefore be more likely to accurately assess their own HIV risk before donating blood.
Regardless of the type of sex people are having, or their number of partners, HIV risk can be virtually eliminated through prevention methods such as consistent and reliable condom usage and taking pre-exposure prophylaxis (PrEP) medication.
We have tools to fight this crisis, but public health and medicine are hampered by systemic bias.
Yet the FDA’s revised guidelines do not take into account these other protective behaviors. In fact, they would continue to require individuals to be off PrEP for three months before donating because of concerns that taking the medication increases your likelihood of getting a false negative HIV test. But a donation policy inclusive of people who regularly take PrEP could help ensure a safer blood supply. And to prevent the mostly hypothetical risk of a false negative HIV test, blood from these potential donors could be individually screened using nucleic acid testing.
The newly proposed rules also continue to specifically defer individuals who have had any type of sex for money and drugs from donating for three months. Though transactional sex is often higher risk for HIV due to decreased condom usage, blanket deferrals without assessment of actual behavioral risk seems based more on prejudice against sex work, substance use and poverty than on evidence.
Generalizations about sex will not truly measure HIV risk — but they will perpetuate stigma. Rather than lean on assumptions, updated FDA guidelines should ask more detailed behavior-based questions that reflect the science of HIV transmission.
Beyond improving the blood donation process, better questionnaires can help educate more Americans about HIV risk. Many schools do not provide comprehensive sexual health education, and even fewer provide information inclusive of LGBTQ health. Donation screenings offer a powerful educational opportunity and can be the first time people carefully assess their own sexual behaviors. They should get the facts on how to accurately evaluate HIV risk and protect themselves, not oversimplifications that may lead to misinformation.
The FDA is clearly trying to create more inclusive and evidence-backed policies. The proposed changes will likely reduce stigma for some Americans and possibly reduce the number of young men who are outed at work and school during blood donation drives.
Yet as a doctor, I’m disappointed by the lack of nuance that still exists in the new donation rules — and I hope to see further changes. As a gay man, I’m relieved to see the FDA work toward ending a ban based on sexual orientation. I look forward to when I can donate blood and contribute to the supply my patients rely on.
Eric Kutscher is a primary care and addiction medicine doctor in New York City.
More to Read
A cure for the common opinion
Get thought-provoking perspectives with our weekly newsletter.
You may occasionally receive promotional content from the Los Angeles Times.