Pfizer COVID-19 vaccine poised to get full FDA approval next week, source says
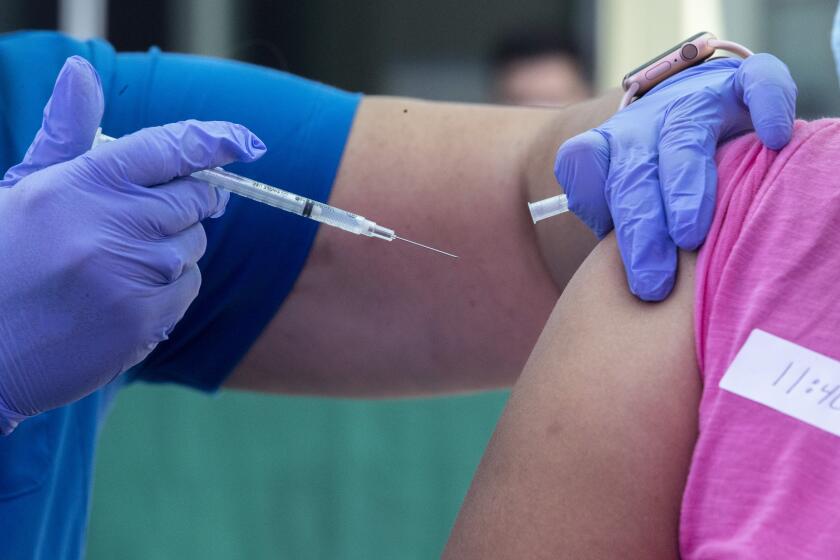
The U.S. Food and Drug Administration is poised to fully approve Pfizer Inc.’s coronavirus vaccine early next week as the Biden administration tries to woo more Americans to get the shot.
The approval probably will come Monday or Tuesday, according to one official familiar with the plans, speaking on the condition of anonymity ahead of the announcement. Pfizer’s vaccine has been in use in the U.S. based on an emergency authorization.
California state officials and private businesses are increasingly prepared to request proof of COVID-19 vaccination as a precondition of both work and play.
The White House referred questions to the FDA, which didn’t immediately respond to requests for comment. The development was first reported by Politico.
Biden has called for approval. He believes it will ease doubts about the vaccine and also give firmer footing for companies and schools to require inoculations, officials familiar with his thinking say.
“My plea is that for those who are not vaccinated: Think about it,” Biden said earlier this month. “God willing, the FDA is going to be coming out in a reasonable timeframe to say this vaccine is totally safe.”
Biden has yet to nominate a permanent head of the FDA. The administration has privately decided against nominating the acting commissioner, Janet Woodcock, people familiar with the plans have said.
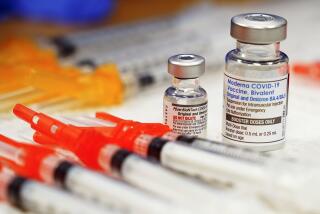
Los Angeles County has begun rolling out third doses of the Pfizer and Moderna COVID-19 vaccines to certain immunocompromised people.