What happens to COVID vaccines and drugs authorized for emergency use when health emergency ends?
On May 11, the central pillar of the country’s pandemic response — the declaration of a national emergency that began March 1, 2020 — will come down. But Americans will continue to have access to the vaccines, drugs and medical devices that were authorized for emergency use against COVID-19, so long as they remain sufficiently safe and effective in the view of the U.S. Food and Drug Administration.
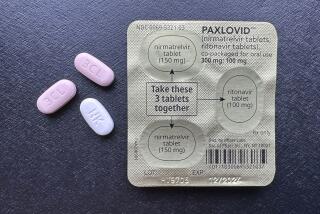
The antiviral medication Paxlovid will not disappear from pharmacy shelves. Children under 12 will still be able to get booster shots of the Pfizer-BioNTech and Moderna vaccines. Adolescents and adults will have the option of getting a dose of the Novavax vaccine.
Coronavirus tests that require you to swab your nose will remain available online and in stores. And myriad lab tests, blood processing devices and specialized pieces of personal protective equipment authorized for use in the pandemic will still be used in commercial labs, research centers and hospitals.
The cost of testing for coronavirus infections will shift for many Americans after May 11. But vaccines will remain free, and antiviral medications purchased by the government will be offered without charge for as long as supplies last.
Surprised that COVID-19 products authorized “for emergency use” are available after the emergency ends? Blame the hurricane of fine print set in motion by the pandemic.
To address the uncontrolled spread of a deadly novel virus, the federal government quickly erected a structure that drew upon a raft of laws on the books.
At least two of those pandemic-response measures were triggered when the secretary of Health and Human Services issued a finding on Jan. 31, 2020, that a public health emergency exists. One is section 319 of the Public Health Service Act, which allowed states to shift federal funds meant for other tasks to their pandemic response. The other, section 564 of the Food, Drug, and Cosmetic Act, gave the FDA power to authorize the use of certain vaccines, drugs and medical devices under an expedited review process.
However, deconstructing a structure built on the fly is a delicate process, and not all the struts can come down at once.
Transitioning out of the COVID emergency phase could eventually spell the end of universal access to free vaccines, treatments and tests.
The pandemic struck hard and fast, and for months, not a single drug, biologic therapy, vaccine or test was available to combat the coronavirus. Under normal circumstances, it can take years for a new medication, vaccine or device to gain the FDA’s blessing to enter the U.S. market. In April 2020, at the peak of the first wave, with more than 2,100 Americans dying of COVID-19 every day, that wasn’t fast enough.
The 1938 law that established the FDA offered a solution. If the secretary for the Department of Health and Human Services makes a declaration that “circumstances exist to justify” it, the FDA is allowed to grant emergency use authorization to badly needed medications, vaccines or devices, allowing them legal access to the U.S. market.
That power will remain in the FDA’s hands until the HHS secretary’s declaration is withdrawn, said Lawrence Gostin, an expert on public health law at Georgetown University.
To win emergency use authorization, or EUA, drug or device makers may present the FDA with less evidence of a product’s safety and effectiveness than is required for full approval. The clinical trials might not last as long or enroll as many people. They might use a simple measure of a product’s risks or benefits as a proxy for a more complex outcome.
In short, a company’s case for an EUA might leave more doubt than usual about the product’s safety, accuracy or effectiveness. But in a national health emergency, the FDA can use that limited data to make an expedited decision on the basis of the “best available” evidence.
Most health plans regulated by the California Department of Managed Health Care will be required to continue paying for at-home COVID test kits.
The notice that granted the FDA this extraordinary power, effective Feb. 4, 2020, appeared in the Federal Register, the daily publication that prints the finest of the fine print of U.S. government operations.
The Federal Register also carried the announcement that then-HHS Secretary Alex Azar had invoked the Public Readiness and Emergency Preparedness Act, effective Feb 4, 2020. The PREP Act gave companies, hospitals and medical professionals who supply or use “countermeasures” authorized for emergency use sweeping protections from liability if those products turn out to cause harm. Those liability protections remain in place until October 1, 2024.
If anyone is harmed, the PREP Act makes them eligible for benefits under the Countermeasures Injury Compensation Program, which is funded by Congress.
Ideally, a product with an EUA will go on to win full FDA approval by meeting the more time-consuming requirements. That has happened for the initial versions of the COVID-19 vaccines Comirnaty (made by Pfizer and BioNTech for those 12 and up) and Spikevax (made by Moderna for adults). The COVID medications Veklury (remdesivir), Actemra (tocilizumab) and Olumiant (baricitinib) are also FDA approved.
Until then, an EUA is strictly provisional. The FDA retains the power to withdraw or alter its EUA in ways large and small, as it sees fit. Sometimes the agency broadens the population of patients for whom a product can be used. Others times it has forbidden the use of a product until further notice — the regulatory fate of a passel of monoclonal antibody treatments that became ineffective against the Omicron variant.
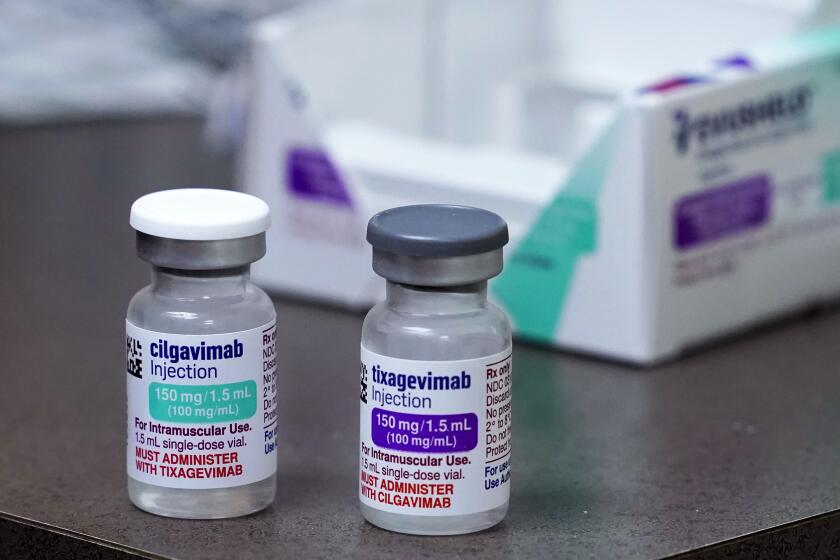
Evusheld was designed to protect immunocompromised people from serious cases of COVID-19, but the latest Omicron variants have rendered the drug useless.
All these vaccines, medications, tests and devices cost money. So far, the tab has been picked up by the federal government.
That won’t come to an abrupt halt when the federal public health emergency ends May 11. But there will be changes.
Medicare patients won’t automatically get eight free coronavirus antigen tests a month, and people insured by Medicare or private insurance may begin to see co-pays for some doctor-ordered testing. But all COVID-19 testing will be free until at least September 2024 for people insured through Medicaid, according to a report by the Kaiser Family Foundation.
For the foreseeable future, vaccines will be offered free of charge. The federal government has purchased 166 million doses of the new bivalent boosters, and the roughly 113 million doses that are unclaimed will continue to be provided for free under virtually all circumstances.
Even after those are exhausted, insurers must offer COVID-19 vaccines without out-of-pocket costs under the Affordable Care Act and other laws.
But for people who must foot the bill themselves, as well as for insurers, an Omicron-targeting dose of Pfizer’s Comirnaty vaccine or Moderna’s Spikevax could more than quadruple in price after government supplies are gone.
Moderna and Pfizer have announced plans to quadruple the price of their COVID vaccines, putting them out of reach for millions. The U.S. should step in.
The government will also continue to bear the cost of fighting new COVID infections — for a while. Some 6 million courses of Paxlovid have been used to treat Americans, and the federal government has about 24 million left. They will be distributed free of charge until stocks are depleted.
When existing inventories are used up, there will be no more free medications or vaccines unless Congress ponies up the cash for additional purchases.
“It’s federal supplies that will dictate when a lot of changes take place,” said Kaiser Family Foundation Vice President Jen Kates.