Russia approves and touts a coronavirus vaccine, but critics are skeptical

MOSCOW — Russia on Tuesday became the first country to officially register a coronavirus vaccine and declare it ready for use, despite international skepticism. President Vladimir Putin said that one of his daughters has already been inoculated.
Putin emphasized that the vaccine underwent the necessary tests and has proven effective, offering a lasting immunity from the coronavirus. However, scientists at home and abroad have been sounding the alarm that the rush to start using the vaccine before Phase 3 trials — which normally last for months and involve thousands of people — could backfire. Other vaccine candidates, including ones being developed in the U.S. and Britain, are undergoing such widespread tests.
Speaking at a government meeting Tuesday, Putin said Russia’s vaccine had proven safe to use. The Health Ministry said in a statement that the vaccine is expected to provide immunity from the coronavirus for up to two years.
“I know it has proven efficient and forms a stable immunity, and I would like to repeat that it has passed all the necessary tests,” Putin said. “We must be grateful to those who made that first step very important for our country and the entire world.”
But U.S. officials were wary of Russia’s claim. “The point is not to be first with a vaccine,” U.S. Health and Human Services Secretary Alex Azar said Tuesday on a visit to Taiwan. “The point is to have a vaccine that is safe and effective for the American people and the people of the world.”
Even Russia’s own Assn. of Clinical Trials Organizations warned against rushing matters. “Fast-tracked approval will not make Russia the leader in the [vaccine] race,” the association said. “It will just expose consumers of the vaccine to unnecessary danger.”
The first wave of coronavirus vaccines might be like a flu shot, experts say, curbing symptoms in some patients but not protecting them from COVID-19.
The vaccine developed by the Gamaleya institute in Moscow uses a different virus — the common cold-causing adenovirus — that’s been modified to carry genes for the “spike” protein that coats the coronavirus, as a way to prime the body to recognize if a real COVID-19 infection comes along. That method is similar to the one in vaccines being developed by China’s CanSino Biologics and Britain’s Oxford University and AstraZeneca.
Advanced clinical trials of the Russian vaccine are set to start Wednesday, Kirill Dmitriev, chief executive of the Russian Direct Investment Fund, told reporters. The fund bankrolled the development of the vaccine.
The trials study will span several countries, including the United Arab Emirates, Saudi Arabia, the Philippines and possibly Brazil, and will involve “several thousand people,” Dmitriev said.
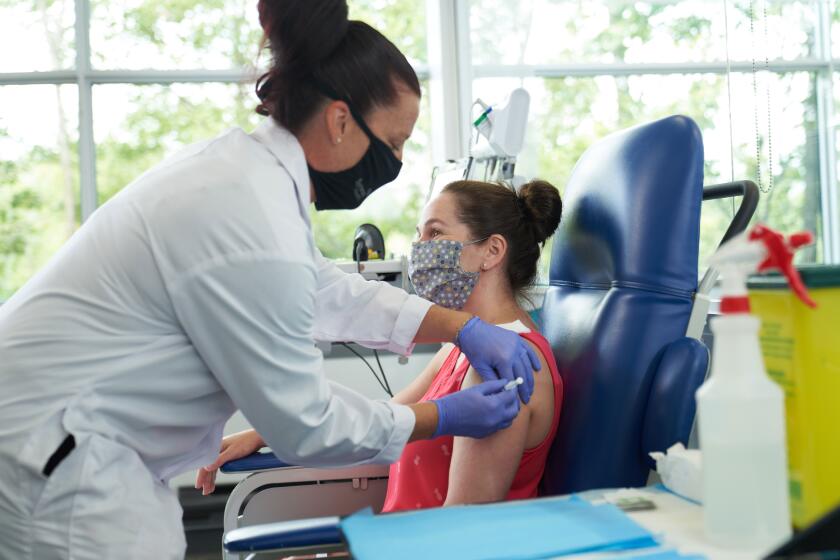
In the meantime, the vaccine will be offered to tens of thousands of others.
Testing vaccines for the coronavirus raises tough ethical questions
“We expect tens of thousands of volunteers to be vaccinated within the next months,” Dmitriev said. “So people outside of clinical trials will have access to the vaccine in August and some, already on the massive scale, in October.”
The Gamaleya’s director, Alexander Gintsburg, said initially that there would be only enough doses to give the vaccine in 10 to 15 of Russia’s 85 regions, according to the Interfax news agency.
So far, Russia has not yet published any scientific data from its first clinical trials, which has fueled skepticism and concern.
“The collateral damage from release of any vaccine that was less than safe and effective would exacerbate our current problems insurmountably,” Danny Altmann, an immunology professor at Imperial College London, said in a statement Tuesday.
Russia boasts that it’s about to be the first country to approve a COVID-19 vaccine, but it’s yet to complete clinical trials, raising concerns.
Putin said that one of his two daughters had received two doses of the Russian-produced vaccine and was feeling well. “She has taken part in the experiment,” he said.
The Russian leader said that his daughter had a temperature of 100.4 degrees on the day of the first vaccine injection, and then it dropped to just over 98.6 degrees the following day. After the second shot she again had a slight increase in temperature, but then that subsided.
“She’s feeling well and has high number of antibodies,” Putin added. He didn’t specify which of his two daughters, Maria or Katerina, received the vaccine.
Russian authorities have said that medical workers, teachers and other risk groups would be the first to be inoculated. Deputy Prime Minister Tatyana Golikova said that the vaccination of doctors could start as early as this month.
China races against the U.S. and others to make the COVID-19 vaccine first, promoting itself as a benevolent vaccinator for the developing world.
Russian officials have said that large-scale production of the vaccine could start in September, and mass vaccination could begin as early as October. Putin emphasized that the vaccination would be voluntary.
Russia has registered 897,599 coronavirus cases, including 15,131 deaths.
When the pandemic struck Russia, Putin ordered state officials to shorten the time of clinical trials for potential coronavirus vaccines.
Becoming the first country in the world to develop a vaccine was a matter of national prestige for the Kremlin as it tries to assert the image of Russia as a global power. State television stations and other media have praised scientists working on it and presented the work as the envy of other nations.
Can a coronavirus vaccine really be developed this year? Dr. Anthony Fauci and other experts say it’s possible but far from certain.
Gintsburg, head of the Gamaleya institute, raised eyebrows in May when he said that he and other researchers had tried the vaccine on themselves.
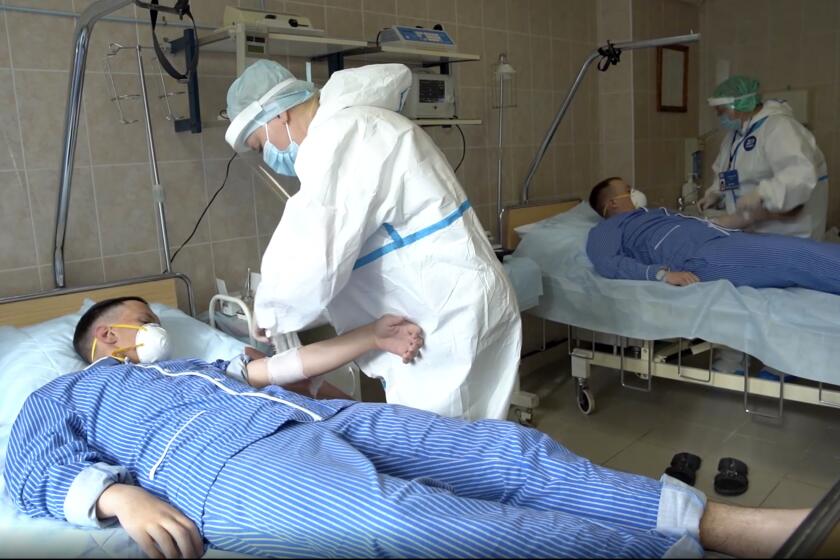
Human studies started June 17 among 76 volunteers. Half were injected with a vaccine in liquid form, and the other half were administered a vaccine that came as soluble powder. Some in the first half were recruited from the military, which raised concerns that servicemen may have been pressured to participate.
Amid Moscow’s rush to become the first to create a vaccine, the U.S., Britain and Canada last month accused Russia of using hackers to steal vaccine research from Western labs.
As the trials were declared completed, questions arose about the vaccine’s safety and effectiveness. Some experts scoffed at Russian authorities’ assurances that the vaccine drug produced the desired immune response and caused no significant side effects, pointing out that such claims need to be backed by published scientific data.
“The U.S. standards are so much more stringent,” White House advisor Kellyanne Conway said Tuesday on “Fox & Friends. “Our FDA in our country sets the standards, and what I understand from the Russia announcement is this is nowhere near where we are.”
The World Health Organization said all vaccine candidates should go through full stages of testing before being rolled out. Experts have warned that vaccines that are not properly tested can cause harm in many ways, from a negative impact on health to creating a false sense of security or undermining trust in vaccinations.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.