FDA approves a new antidepressant: Brintellix
The Food and Drug Administration late Monday approved a new antidepressant medication that is a novel variant on the selective serotonin reuptake inhibitors, or SSRIs, that have become the mainstay of depression treatment. The drug, whose generic chemical name is vortioxetine, is to be marketed under the commercial name Brintellix.
Brintellix received the agency’s approval without deliberation by a science advisory panel, a public review of an investigative drug’s safety and effectiveness. In announcing its decision, the FDA said the new medication was found “effective in treating depression” in six clinical trials that compared outcomes in subjects taking the drug against those of subjects who received a sham medication, or placebo.
The agency also cited a clinical trial that found among those taking Brintellix a decreased likelihood of becoming clinically depressed after successful treatment for an earlier episode.
Like SSRIs and a range of older antidepressant medications, Brintellix will carry a boxed warning alerting patients and physicians that with children, adolescents and young adults between 18 and 24, antidepressants can increase the risk of suicidal thoughts and behavior.
“Since medications affect everyone differently, it is important to have a variety of treatment options available for patients who suffer from depression,” said Dr. Mitchell Mathis, acting director of the Center for Drug Evaluation and Research’s division of psychiatry at the FDA.
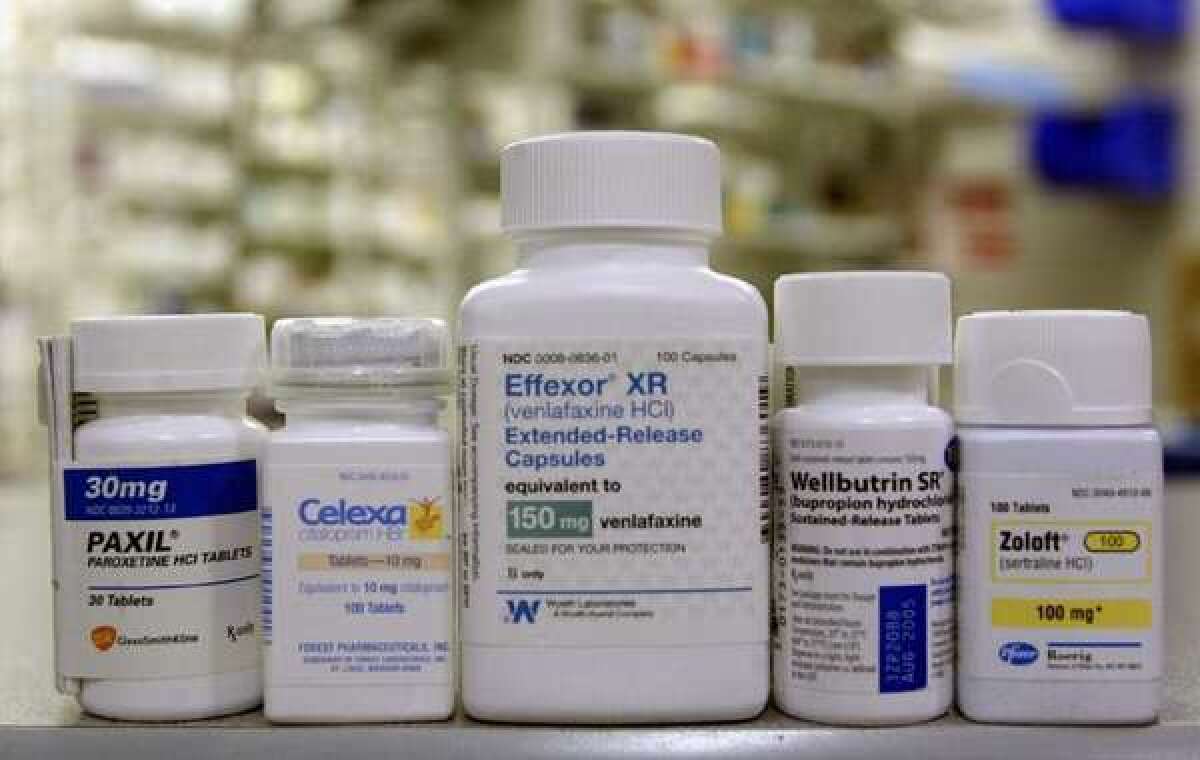
At a time when most top-selling antidepressant medications have become available in cheaper, generic formulations, the latest approval brings a new brand-name depression medication to the American prescription marketplace. Brintellix is co-marketed by the Japanese firm Takeda Pharmaceuticals and the Danish pharmaceutical company Lundbeck.
Not included in the FDA’s announcement were benefits the drug’s developers had hoped to tout to potential prescribers and patients. As a “serotonin modulator and stimulator,” vortioxetine influences the availability of the neurotransmitter serotonin by five different mechanisms. The developers had argued that by increasing its modes of action above and beyond those of standard SSRI medications known by such commercial names as Prozac, Lexapro, Zoloft and Paxil, this new medication might represent an improvement on those drugs.
The drug developer submitted early research - conducted on rats - that suggested vortioxetine may improve some dimensions of memory. A trial in human subjects showed that those taking vortioxetine had better cognitive function than those taking the antidepressant duloxetine (marketed as Cymbalta). A single clinical trial found that compared with subjects taking the antidepressant venlafaxine (marketed as Effexor), those taking vortioxetine suffered less sexual dysfunction.
In all those cases, the FDA declined to approve such claims for the new drug until they were replicated and extended by further research.
“These hopes, of course, remain to be proven,” said Dr. Michael Thase, a University of Pennsylvania psychiatrist and psychopharmacologist who consulted on the development of the drug. “It is different enough” from the welter of SSRIs currently available “that it’s not simply a ‘me too’ drug,” he added.
“At the least, you will have a drug with some different effects - different ways in which it interacts with the brain” to ease depression, he said. “At the most, it will be useful and will become one of our favorite antidepressants. But that typically takes several years to evolve.”