Q&A: The fascinating backstory of the periodic table, which is about to turn 150 years old
The periodic table has become an icon of science. Its rows and columns provide a tidy way of showcasing the elements — the ingredients that make up the universe.
It seems obvious today, but it wasn’t to generations of early chemists. That changed when Dmitri Mendeleev started writing a textbook and pondered ways to group the elements together in order to lighten his load.
The Russian chemist spotted an elegant and powerful pattern: He recognized that certain elements exhibited similar traits, and that these traits varied regularly — or periodically — with increasing atomic weight.
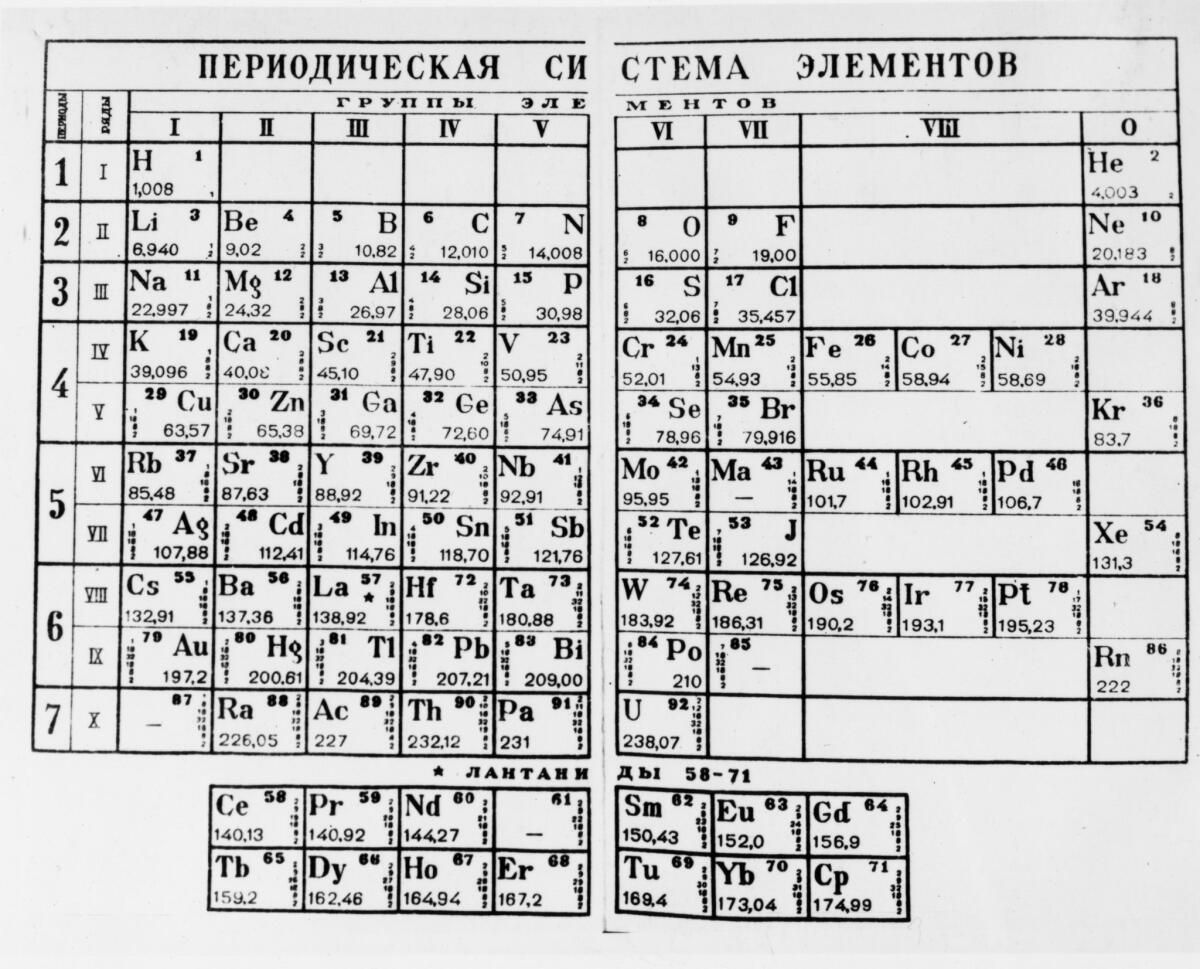
So on Feb. 17, 1869 (according to the Julian calendar used in Russia at that time), Mendeleev published a chart of the 60-odd elements known at the time, sorted by their weights and properties. He called it “An Attempt at a System of Elements, Based on Their Atomic Weight and Chemical Affinity.”
It has come to be regarded as one of the greatest scientific contributions of all time.
That’s why the United Nations and the International Union of Pure and Applied Chemistry are celebrating the 150th anniversary of Mendeleev’s periodic table. The festivities kicked off in Paris on Tuesday.
The journal Science is marking the occasion with a special issue about the table, which includes an essay on its origins by Michael Gordin, a science historian at Princeton University. Gordin spoke with The Times about Mendeleev’s invention and its scientific legacy.
Why has the periodic table endured for 150 years?
It’s an amazing tool that can compress a huge amount of information into one format. It’s one of the first things people learn about chemistry. It’s in every textbook. It’s on the wall of pretty much every chemistry classroom in the world.
How did it come about?
Dmitri Mendeleev was writing a textbook when he came up with the idea. It was a scheme he put together in order to help organize the elements in families so that he didn’t have to spend time doing each element individually.
How did it work?
The table is organized by increasing atomic weight, but broken into rows. When Mendeleev did that, he saw that certain elements have very similar properties — they form acids that have similar strength, they form crystals that look the same.
So, in addition to increasing atomic weight, he saw that there is some other pattern that repeats. He’s the one who invented the term “periodic.”
Did he realize he was onto something big?
I think he perceived a pattern that could be one of two things. Is it just a convenient teaching tool, or is it a deep pattern in nature? He saw it both ways.
Over time, he became more convinced that he had discovered a law of nature. That’s normal for scientific findings — when you first propose something, you don’t know it’s true yet.
Would you say Mendeleev “discovered” the modern periodic table?
I usually say that Mendeleev formulated or invented the table. The reason I don’t say discovered is that it’s not like it’s a rock or a mountain. It’s a set of relations among things that are like rocks or mountains.
But he wasn’t the only chemist working on a table 150 years ago, was he?
That’s right. There were six different formulations of the table in the 1860s.
What sets his table apart?
The first thing is that Mendeleev does all the elements. Previous people had put in many or most, but hadn’t done all of them because they weren’t sure about the atomic weights. Mendeleev made guesses about their weights to fit them in the table.
The second thing is that he predicted the existence of new elements. When those elements are discovered, his table stands out.
The third reason is that he was very insistent during his lifetime that he deserved credit for the periodic table.
Let’s back up a minute. How did Mendeleev predict the existence of undiscovered elements?
There are several hypotheses by historians because he didn’t write down his thought process.
When Mendeleev started lining up elements with similar properties into columns, he noticed that, in some places, an element seemed to be in the wrong place and should be one column over. When he moved it over, everything worked out. But then there’s an empty gap. And he’s like, “OK, how do I explain what’s in the gap?”
And how did he explain it?
He said, “Well, its atomic weight should be about this, because I can average from the elements around it and guess it.” And he’s like, “I know what its crystal structure should be. And I know something about what kinds of acids it would form, because it has those properties of the elements above and below it.”
Within 15 years, three of the elements he predicted in detail were discovered. And they had exactly the properties he said they would.
So other chemists didn’t make predictions?
Other people recognized that there was a pattern. But they didn’t guess about the properties of the elements.
Mendeleev’s main competitor is a German named Lothar Meyer. Meyer made a few versions of a similar table in the 1860s, but he never made predictions. He thought it was reckless to guess. He’s like, “Chemists shouldn’t be doing that, because we don’t yet know enough.”
What was going on in the 1860s that gave everyone the idea to make tables of the elements?
Today, the periodic table is organized by atomic number, which is the number of protons in the nucleus. But they didn’t know about protons then, so they organized everything by atomic weight. They had what they thought were accurate atomic weights for all the elements, but some of those weights were measured by different systems.
Then, in 1860, there was an international meeting of chemists in Germany. Mendeleev happened to be there because he was studying nearby. They propose a unified way of organizing the atomic weights, and when they do that, they correct a whole bunch of atomic weights.
Within a year or two, people started seeing these patterns.
Were there earlier attempts to organize the elements?
Yes. One method was alphabetical, which was very common.
The other organized elements by their ability to bond together. So you would create a table and say, sulfur bonds really well with this and this and this, in this order. They look like periodic tables, but they are totally different. They don’t list all of the elements, and things appear several times.
Do we know what kind of guy Mendeleev was?
We actually know a lot about him. He was born in a Siberian city called Tobolsk, which is almost in the exact center of Russia. He ended up in St. Petersburg for education, and he stayed there most of his life — he taught at the University of St. Petersburg — but traveled very broadly.
He was very boisterous, kind of funny, quick to lose his temper, but also clearly very charismatic and engaging. He was also very politically active. He was in the papers a lot.
After he finished the table, he decided he would start keeping all of his mail and all of his letters because he knew he would be famous. He’s the kind of person who cared about his legacy and thought of himself quite well.
Did he become famous in his lifetime?
Yes. He did the table when he was 35, in 1869, and he lived until 1907. The table became more and more central to chemistry over the course of his life, so he became internationally well known.
Scientists didn’t really understand atoms until after Mendeleev died. How did that change the table?
We now organize the table based on quantum theory — on the positions the electrons in the outer shell of an atom have. That explains their chemical properties because the electrons determine how they bind with other elements.
Mendeleev didn’t know any of that. The electron was discovered in 1897, and he didn’t like that idea. He didn’t like many of these new ideas. When people discovered new things he couldn’t put in the table, he got very frustrated. It bothered him.
But later, Niels Bohr, the Danish physicist who’s one of the architects of quantum theory, published a very interesting version of the periodic table that incorporates the insights of the quantum vision of the atom to help explain how the system works. It silenced those who thought the table was just a lucky guess.
What else has changed over the last 150 years?
All of these new elements that have been discovered, the very heavy elements. They are principally results of using colliders and things like that to make them. They live for a very short period of time — microseconds. But filling out the table has taught us a great deal about how the nuclei of these atoms work.
We now understand why there are as many columns in the table as there are, and how many rows down we can go before the atom becomes too unstable. We now have a table with no gaps, and that gives us a real feeling of understanding nature.
Is the table complete now?
I’m sure somebody will try to create new elements so you could build further rows. But the question of whether it’s worth the investment for the amount of knowledge we get is a question that scientists and politicians would have to answer. It’s not my business to decide.
Is there anything about the modern periodic table that would have surprised Mendeleev?
I think he would have been really surprised at how heavy the elements can get, that we’ve been able to get to 118.
But I think he would have been very gratified that the table is still omnipresent — that this thing he did is still around.
This interview was edited for length and clarity.